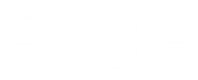Show this safety data sheet to the doctor in attendance. This means that we will split them apart in the net ionic equation.

Is Hno3 An Acid Or Base Strong Vs Weak Nitric Acid
/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png)
List Of Common Strong And Weak Acids
Nitric Acid Reactions Hno3
Normal salt acid salt basic salt and double.

Is nitric acid a strong acid. This mineral acid has a variety of applications and presents several health hazards if used without the necessary safety precautions. Symptoms may or may not be delayed. Most commercially available nitric acid has a concentration of 68 in water.
For sulfuric acid which is diprotic the. Strong acids and strong bases are considered strong electrolytes and will dissociate completely. Nitric acid is a very strong acid turns blue litmus red.
You will see lot of reactions of HNO 3 in this tutorial. 4HNO 3 4NO 2 O 2 2H 2 O. The pure compound of nitric acid is colorless and has a suffocating odor.
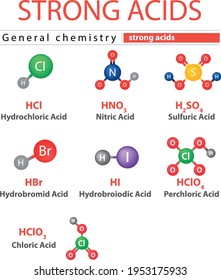
The seven most common strong acids are hydrochloric acid nitric acid sulfuric acid hydrobromic acid hydroiodic acid perchloric acid and chloric acid. H2 acts as a base to abstract an H from the nitric acid. The rest of the acids except for those 6 acids are weak acids.
Basic salts are formed when a strong base reacts with a weak acid. Dangerous fire risk in contact with organic materials. Salts can be acidic basic or neutral.
About 70 percent of the nitric acid produced is consumed as an intermediate in the manufacture of. When a strong acid reacts with a strong base neutral salts are formed. It is toxic and can cause severe burns.
The hydrogen and halogen combine in a reaction to form a weak acid namely hydrofluoric acid while hydrochloric acid is a very strong and extremely powerful and also corrosive in nature but stated as weak acid. It is made up of three elements hydrogen oxygen and nitrogen. Nitrogen dioxide forms acid on contact with moisture and is a poisonous choking gas.
The lower the pH value the higher the concentration of hydrogen ions in the solution therefore a stronger acid. It is a simple diatomic molecule. Strong acids yield weak conjugate bases.
With a total capacity of 11 million tons of HNO 3 per year. Being part of the list of strong acids doesnt give any indication of how dangerous or damaging an acid is though. First-aid measures General Advice Immediate medical attention is required.
The reaction of Nitric acid and Sodium hydroxide represents a net ionic equation involving a strong acid and strong base. This is the reason why it becomes brownish over time though fresh nitric acid is colourless. The different types of salts are.
Hydrochloric acid is an inorganic chemical. Answer 1 of 13. There are 7 strong acids.
Highly toxic by inhalation corrosive to skin and mucous membranes strong oxidizer. The hydrogen and chlorine atom are connected with a. Acid strength is the tendency of an acid symbolised by the chemical formula to dissociate into a proton and an anion The dissociation of a strong acid in solution is effectively complete except in its most concentrated solutions.
Nitric acid 65 - 70 Revision Date 25-Apr-2019 4. Learn more about the properties and uses of nitric acid in this article. PH and pKa of a Strong Acid.
Strong acids like hydrochloric acid HCl have a pH around 0 to 1. Continued exposure to the vapor mist of nitric acid may result in a chronic bronchitis more severe exposure results in. Nitric acid is a strong oxidizing agent with the chemical formula HNO 3.
Weak Acids-A weak acid partly dissociates ions in water. Eye Contact Rinse immediately with plenty of water also under the eyelids for at least 15 minutes. Attacks almost all metals.
When hydrogen chloride is dissolved in water HCl is formed. The resulting H3O hydronium is the conjug. HNO3 H2O H3O NO3 Nitric acid is a relatively strong acid meaning it tends to ionize almost completely in aqueous solution.
Concentrated nitric acid is a strong acid and a strongly oxidizing acid. Immediate medical attention is required. Commonly used in fertilizers and in rare occasions even explosives Nitric acid is a colorless or yellow liquid with a characteristically acrid caustic odor and corrosive properties.
Nitric acid liberates hydrogen gas with metals above hydrogen in the metal activity series. A strong acid is one which completely dissociates in its solvent. What is Nitric Acid.
A strong acid ionizes completely in an aqueous solution by losing one proton according to the following equation. Acidic salts are formed when a strong acid reacts with a weak base. Ingestion causes burning and corrosion of internal tissues 1 If you spill concentrated nitric acid on your skin you should wash it off immediately with a large quantity of water.
The pure compound is colorless but older samples tend to acquire a yellow cast due to decomposition into oxides of nitrogen and water. Nitric acid decomposes on standing to form brown nitrogen dioxide. Exposure to high concentrations of nitric acid vapor may cause pneuomonitis and pulmonary edema which may be fatal.
Nitric Acid Reactions HNO 3 Reactions. The caustic colorless liquid is typically produced on an industrial scale using highly specialized chemical processes and equipment. Under most definitions the acid dissociates into a positively-charged hydrogen ion proton and a negatively-charged anion.
The strong acids and bases are simply those that completely dissociate in water. Strong acid may also be made indirectly from the weak acid by using extractive distillation with a dehydrating agent. Nitric acid is a strong acid and reacts in different ways like a oxidizing reagent oxidizing acid and more with elements and compounds.
It is a strong corrosive acid with a chemical formula HCl. Examples of strong acids are hydrochloric acid perchloric acid nitric acid and sulfuric acid. Its reactions vary on concentration of the nitric acid solution.
Concentrated nitric acid 69 to 71 is a transparent colorless or yellowish fuming suffocating hygroscopic corrosive liquid. Nitric acid colorless fuming and highly corrosive liquid that is a common laboratory reagent and an important industrial chemical for the manufacture of fertilizers and explosives. Nitric acid with water reacts as follows.
It is also known as hydrogen chloride or muriatic acid. When an acid and a base react with each other the products that are formed is a salt an ionic compound that is formed from a reaction between an acid and a base and water. 88 Nitric Acid 881 General 1-2 In 1991 there were approximately 65 nitric acid HNO 3 manufacturing plants in the U.
Nitric acid H NO 3 also known as aqua fortis Latin for strong water and spirit of niter is a highly corrosive mineral acid. Miscible with water and decomposes in alcohol. Nitric acid concentration processes use a dehydrating agent such as sulfuric acid or magnesium nitrate to enhance the volatility of HNO3 so that distillation methods can surpass the azeotropic concentration of nitric acid.
LatexHA aq rightarrow H aq A-aqlatex where HA is a protonated acid H is the free acidic proton and A is the conjugate base. Nitric acid has the chemical formula HNO3 and Calcium Hydroxide has the chemical formula CaOH2. Chloric acid hydrobromic acid hydrochloric acid hydroiodic acid nitric acid perchloric acid and sulfuric acid.
The plants range in size from 6000 to 700000 tons per year. Strong acids have a large value of Ka acid dissociation constant and a small value of pKa logarithmic acid dissociation constant. It is used in the manufacture of various organic and inorganic nitrates.
Nitric acid is a type of potent mineral acid used to make things like fertilizers dyes and high explosives. A strong acid is one that is completely dissociated or ionized in an aqueous solutionIt is a chemical species with a high capacity to lose a proton H In water a strong acid loses one proton which is captured by water to form the hydronium ion. Answer 1 of 11.
Which One Is Stronger Acid Hno3 Vs H2so4 Youtube
Which Is Stronger Sulfuric Acid Or Nitric Acid And Why Quora

Strong Acid Images Stock Photos Vectors Shutterstock
Nitric Acid Images Stock Photos Vectors Shutterstock

Lo I Understand The Difference Between Strong And Weak Acids Ppt Download
Ionization Of Acids A Weak And Strong Acids Acids Differ Enormously In The Extent To Which They Dissociate Into Ions In Aqueous Solution Some Acids Such As Hydrochloric And Nitric Acids Are Strong Electrolytes Completely Dissociated Into Ions These Acids
Solved 8 Nitric Acid Is A Strong Acid This Means That A Chegg Com
What Are Some Examples Of Strong And Weak Acids And Bases Quora